NOGGO S15 - FraStROC
Study for patients with recurrent or relapsed ovarian cancer.
STUDY FOR PATIENTS WITH RECURRENT OR RELAPSED OVARIAN CANCER
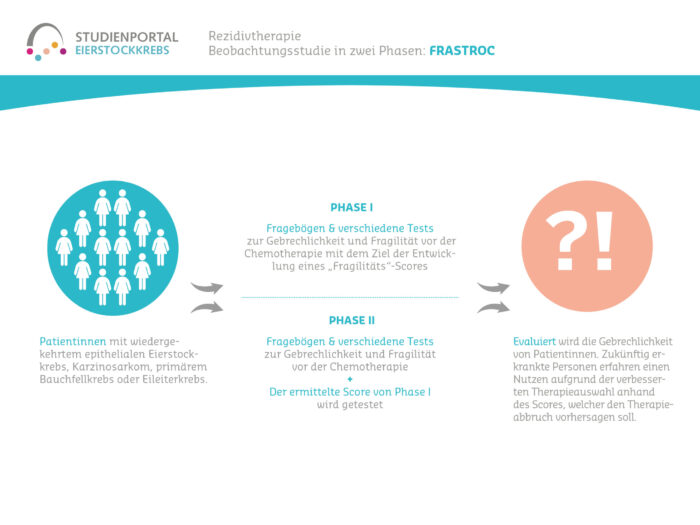
The FraStROC study is a non-interventional, prospective, multicenter observational study in which the frailty and fragility of ovarian cancer patients in the recurrence situation during chemotherapy is evaluated. The study is being conducted in two phases.
What is the aim of the study?
The aim of the study is to prospectively evaluate inflammatory markers, organ dysfunction, functional tests and geriatric assessment measures and patient-reported outcome for their ability to predict hematologic toxicity or non-hematologic toxicity.
What is the procedure for the study?
This is a prospective, multicenter, non-interventional, observational study in 2 stages for patients with epithelial ovarian cancer or carcinosarcoma (EOC), fallopian tube cancer (FTC) or primary peritoneal cancer (PPC) who require monotherapy for recurrent disease and have participated in at least one prior systemic treatment.
Steps 1 and 2:
In the study, demographic, clinical and pathological aspects of the patients are assessed, the measures taken before the start of chemotherapy are documented and an assessment of toxicity is carried out for one year from the start of treatment (follow-up).
In the first step of the study, a frailty score is to be determined on the basis of the data collected in order to estimate the probability that chemotherapy will have to be discontinued due to side effects. In the second step of the study, the calculated score will be tested (validated).
Are there any risks?
No, it is an observational study.
PARTICIPATION REQUIREMENTS
Can I take part in this study?
Most important inclusion and exclusion criteria:
Patients must submit a written declaration of consent in advance and be at least 18 years old:
- have a histologically confirmed diagnosis of epithelial ovarian cancer, carcinosarcoma, primary peritoneal cancer or fallopian tube cancer
- have a recurring cancer
- are eligible for monochemotherapy with paclitaxel, doxorubicin (PLD), topotecan or treosulfan with optional use of bevacizumab
- have received at least one previous treatment regimen for ovarian cancer (permitted from the second line of treatment)
- have a life expectancy of at least 12 weeks.
Ineligible patients:
Patients are excluded if:
- they are only eligible for combination chemotherapy or maintenance therapy after previous chemotherapy or radiotherapy is planned.
In addition, there are further criteria that must be met. Interested patients should speak to an investigator at a study center who can check whether they are eligible for this study.
ARE YOU INTERESTED IN THE STUDY?
Please contact your medical team (gynecologist or oncologist) with this PDF. They will check whether the study is suitable for you and refer you to a study center.
