AGO-OVAR 27 - WoO
WoO: Window of Opportunity study on olaparib and durvalumab in histologically proven EOC
Recruitment for cohort A of the AGO-ovar 27 study was completed in January 2025. Recruitment for cohort B is currently still ongoing.
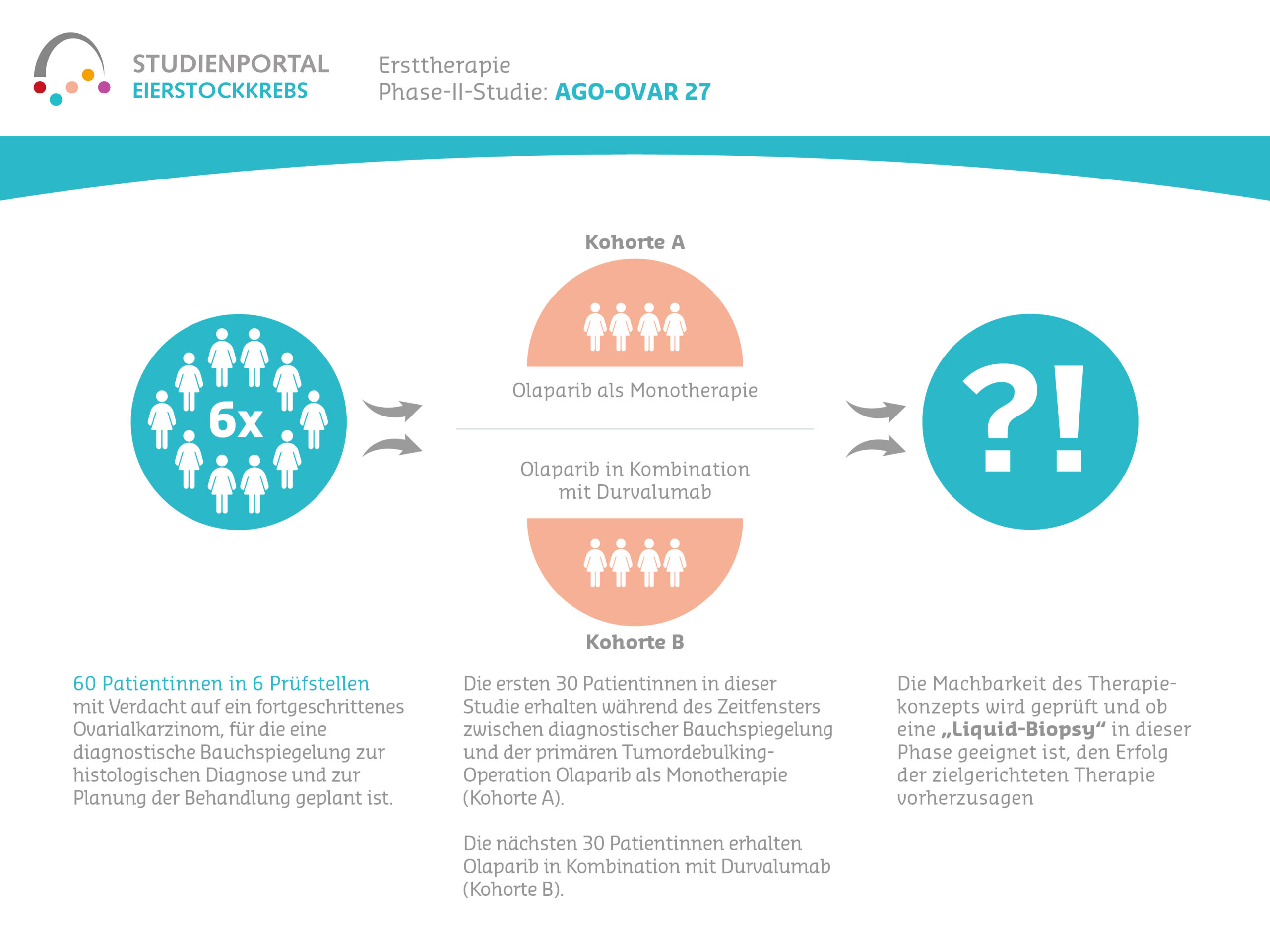
Window-of-opportunity – AGO-OVAR 27 is a non-randomized, open-label phase II feasibility study on the administration of olaparib as monotherapy (cohort A) or in combination with durvalumab (cohort B) prior to primary tumor debulking surgery in histologically proven high-grade epithelial ovarian cancer (EOC).
What is being investigated in this study?
This is a non-randomized, open-label phase II trial investigating the feasibility of treatment with olaparib as monotherapy or in combination with durvalumab during the window of opportunity between the suspected diagnosis or diagnostic laparoscopy and the primary surgery to remove the tumor. In addition, it will be investigated whether changes in the profile of circulating tumor DNA (ctDNA) during this treatment phase are suitable for predicting targeted therapy for individual patients.
What is being investigated in this study?
This is a non-randomized, open-label phase II trial investigating the feasibility of treatment with olaparib as monotherapy or in combination with durvalumab during the window of opportunity between the suspected diagnosis or diagnostic laparoscopy and the primary surgery to remove the tumor. In addition, it will be investigated whether changes in the profile of circulating tumor DNA (ctDNA) during this treatment phase are suitable for predicting targeted therapy for individual patients.
What is the aim of the study?
This study aims to investigate the feasibility of treatment with olaparib as monotherapy or in combination with durvalumab during the window of opportunity between diagnostic laparoscopy and primary surgery for tumor removal. In addition, it will be investigated whether changes in the ctDNA profile during this treatment phase are suitable for predicting targeted therapy for individual patients.
What is the procedure for the study?
The study will take place exclusively at German trial sites. 60 patients (30 per cohort) will be included in the study. The first 30 patients in this study will receive olaparib as monotherapy during the time window between diagnostic laparoscopy and primary tumor debulking surgery (cohort A). The next 30 patients will receive olaparib in combination with durvalumab (cohort B).
Patients receive olaparib as film-coated tablets for oral administration. The dose is 300 mg (2 x 150 mg tablets) twice daily in the morning and evening. The duration of olaparib monotherapy in cohort A is 3 weeks. The duration of olaparib combination therapy with durvalumab in cohort B is 4 weeks.
Patients receive durvalumab via a 60-minute infusion (drip) directly into the vein on day 1 of the four-week combination therapy of cohort B in a single dose of 1500 mg.
Possible maintenance therapy begins after completion of chemotherapy. Only if no other maintenance therapy is available according to the guideline standard can patients with negative BRCA and HRD status be treated with olaparib as an investigational drug for maintenance therapy for a maximum of 24 months.
Patients in cohort B can already receive durvalumab as an investigational drug during the
chemotherapy phase. In the subsequent maintenance phase, durvalumab can be given either in
combination with olaparib or alone as an investigational drug for a total of up to 24 months
, provided the patients are suitable for this.
Are there any risks?
You will be informed about possible risks and side effects associated with participation during an information session.
Conditions of participation
Women aged 18 and over can take part in this study,
- with suspected advanced ovarian cancer for whom a diagnostic laparoscopy is planned for histological diagnosis and treatment planning
- who are willing and able to provide tumor biopsies from diagnostic laparoscopy and primary surgery for tumor removal
- with adequate organ and bone marrow function and normal blood pressure
- with an ECOG performance status (quality of life index of the Eastern Cooperative Oncology Group) of 0-1
However, there are also other criteria that must be met in order to participate in the study. Interested patients should speak to an investigator at a study center who can check whether they are eligible for this study.
Where can I take part in the studies?
