NOGGO ov54 - Scout 1
Observational study to collect data from the everyday routine of patients with ovarian cancer undergoing initial therapy
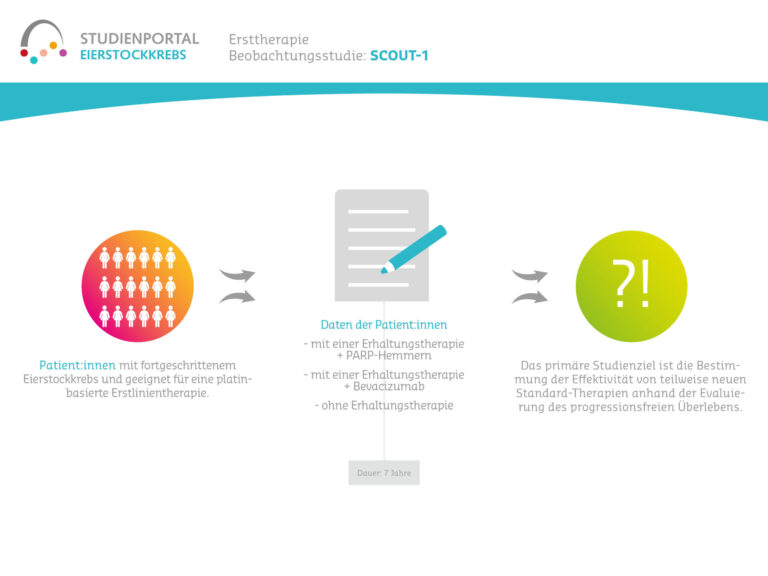
SCOUT-1 is a non-interventional study, a so-called observational study with the aim of collecting clinical and patient-reported data from the daily routine of patients with ovarian cancer. Eligible patients are those who are suitable for platinum-based first-line chemotherapy and for whom BRCA/HRD testing is planned. The observational study will collect data over a period of 7 years, but will not interfere with the course of treatment.
What is being investigated in this study?
The majority of patients with ovarian cancer are diagnosed at an advanced stage. Despite improved results from debulking surgery (removal of the tumor tissue) and a good response to platinum-based first-line chemotherapy, most patients with ovarian cancer suffer a recurrence within two years. (Source: S3 guideline Diagnostics, therapy and aftercare of malignant ovarian tumors, as of May 2022: Guideline program)
In the last two years, new treatment options have been researched, particularly in follow-up care, and expanded to include the concept of maintenance therapy. This has improved progression-free survival (i.e. the time between participation in a trial and disease progression). For example, the effectiveness of PARP inhibitors has been proven in advanced ovarian cancer and BRCA mutations. PARP inhibitors disrupt the DNA repair of cancer cells and thus activate the natural self-destruction program. A BRCA mutation is altered genetic material that can promote cancer. Studies have also shown that PARP inhibitors are effective in patients without a BRCA mutation.
Based on these new findings, a strong recommendation was made for the use of PARP inhibitors in first-line maintenance therapy. This extension of the approval of PARP inhibitors may influence diagnostic and therapeutic measures, follow-up care and patients’ perceptions and needs in everyday care. Most of these aspects will be observed in the study.
What is the aim of the study?
The primary objective of the study is to document data on disease progression and treatment of newly diagnosed advanced ovarian cancer. These data are collected during routine treatment. The aim is to determine the effectiveness of some new standard therapies by evaluating progression-free survival.
This observational study will also provide new insights into the treatment patterns and outcomes of patients with ovarian cancer under everyday conditions in Germany. The focus is on the effectiveness, expectations and needs of patients, molecular test procedures, selection criteria and tolerability of standard treatment sequences. Patient questionnaires, which patients fill out themselves on their state of health and quality of life, are intended to better understand the influence of the disease and treatment over the course of the disease.
What is the procedure for the study?
Patients are observed for up to seven years. During routine visits to the doctor, data on the patient’s medical history and current therapy is documented and some of this data is transferred to the study database. Therapy data collected in the years following the first-line therapy will also be transferred. Participation in the study does not result in any additional visits to the doctor. The quality of life and preference data are recorded electronically by the patients themselves using a questionnaire, so a smartphone, tablet or computer is required for participation.
Are there any risks?
In the SCOUT-1 observational study, there are no additional medical risks outside of routine care. There are confidentiality risks associated with any collection, storage, use and transmission of data. These risks cannot be completely ruled out and increase the more data can be linked together. The sponsor of the study does everything possible to protect the study data according to the state of the art.
Conditions of participation
Women aged 18 and over can take part in this study:
- with newly diagnosed advanced ovarian cancer
- suitable for platinum-based first-line therapy
- with a completed or planned BRCA/HRD test
However, there are also other criteria that must be met in order to participate in the study. Interested patients should speak to the investigators at a study center, who can check whether this study is suitable for them.
Where can I take part in the study?
This study is supported by:

