NIS Niraparib - NOGGO-ov45 - CAROLIN
For patients with platinum-sensitive ovarian cancer who have no contraindications to niraparib
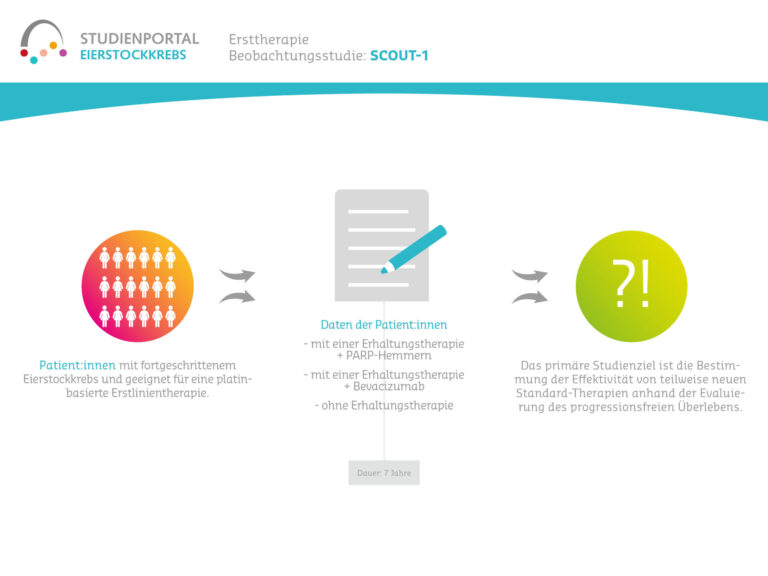
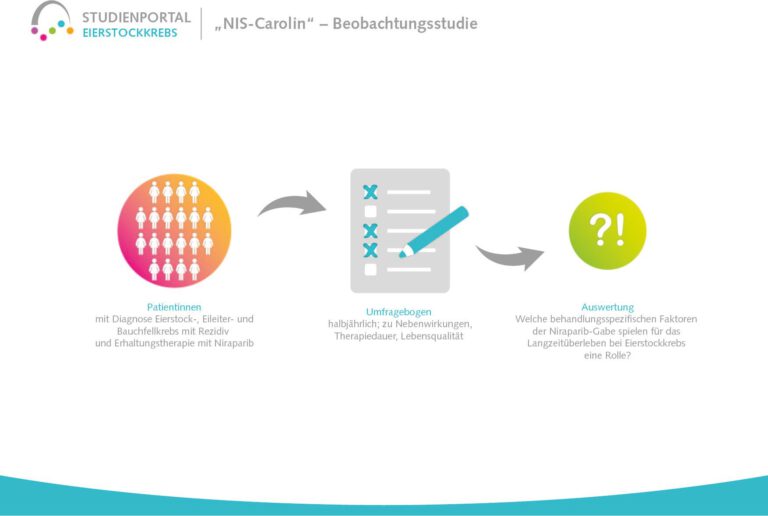
This non-interventional observational study aims to investigate the long-term treatment with niraparib in patients with platinum-sensitive ovarian cancer who have been diagnosed for the first time or have already suffered a relapse (recurrence) and to identify factors associated with long-term survival. Niraparib is a so-called PARP inhibitor that prolongs the effect of chemotherapy after its completion by suppressing the repair mechanisms in the cancer cells damaged by the chemotherapy. The damaged cells are then unable to regenerate and die.
What is the aim of the study?
The aim is to find out which typical features/characteristics with regard to the disease, therapy and patients are associated with long-term survival. The study will also investigate which treatment-specific factors of niraparib administration (dose, side effects, duration of therapy, quality of life during therapy) correlate with long-term survival.
What is the procedure for the study?
This is an observational study in which the decision to treat with niraparib was made by the doctor and patient before the start of the study and the therapy is carried out according to the standard regimen. Patient data is collected at the beginning of the study and every six months thereafter, for up to eight years after study inclusion.
Are there any risks?
This is a purely observational study, i.e. the patients do not receive any additional medication or other treatment regimes. In this respect, the study participants are not exposed to any additional risks or side effects. However, treatment with niraparib, which is prescribed to patients with ovarian cancer after chemotherapy in the relapse situation, can have side effects, e.g. lead to a lack of blood platelets, a lack of certain white blood cells (neutrophil granulocytes), anemia, high blood pressure or fatique syndrome.
Participation requirements
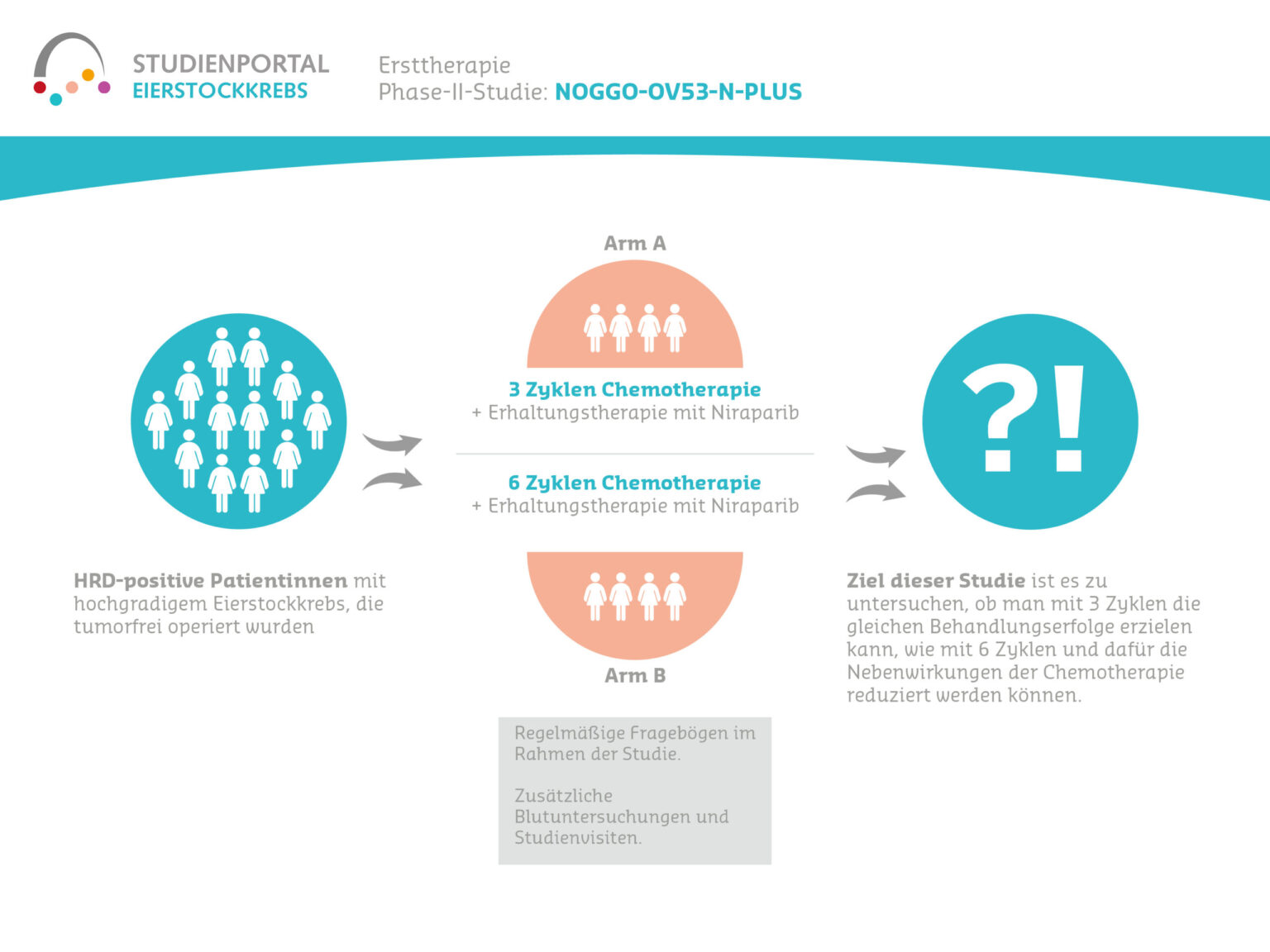
Women aged 18 and over with ovarian, fallopian tube or peritoneal cancer who have been diagnosed for the first time or have relapsed (confirmed by histological findings) and who have achieved a tumor response with chemotherapy can take part in this study. Patients must be suitable for maintenance therapy with niraparib, i.e. they must have no contraindications and be able to take oral medication independently and reliably. Patients who are hypersensitive to the preparation, pregnant or breastfeeding may not take part in the study.
Where can I take part in the study?
This study is supported by:
The research-based pharmaceutical company GLAXOSMITHKLINE is a partner in this study.