NOGGO S30/ Expression XVIII (18)
INTERNATIONAL SURVEY ON THE IMPACT OF CRISES ON PATIENTS WITH GYNECOLOGICAL TUMORS
Anxiety is a central issue in cancer patients with gynecologic diseases and can be exacerbated by various crises, such as war, finances and pandemics. Although clinical cancer research is mainly focused on therapies, several studies emphasize the need to consider fear in communication between patients, physicians and medical staff. The results of this survey will be used to identify potential solutions to overcome conflicts and help patients communicate with healthcare professionals and their families about these issues. This is a global study being conducted for the first time and includes patients from all continents to gain a global perspective.
What is the aim of the survey?
The aim of this survey is to establish a relationship between recurring anxiety and other crises (wars, financial and family crises, environmental disasters and pandemics) and to bring the anxieties together in order to draw clinical conclusions and analyze the interplay and possible solutions to overcome these conflicts. The results will then be used to develop and provide specific information materials and offer better mental health counseling.
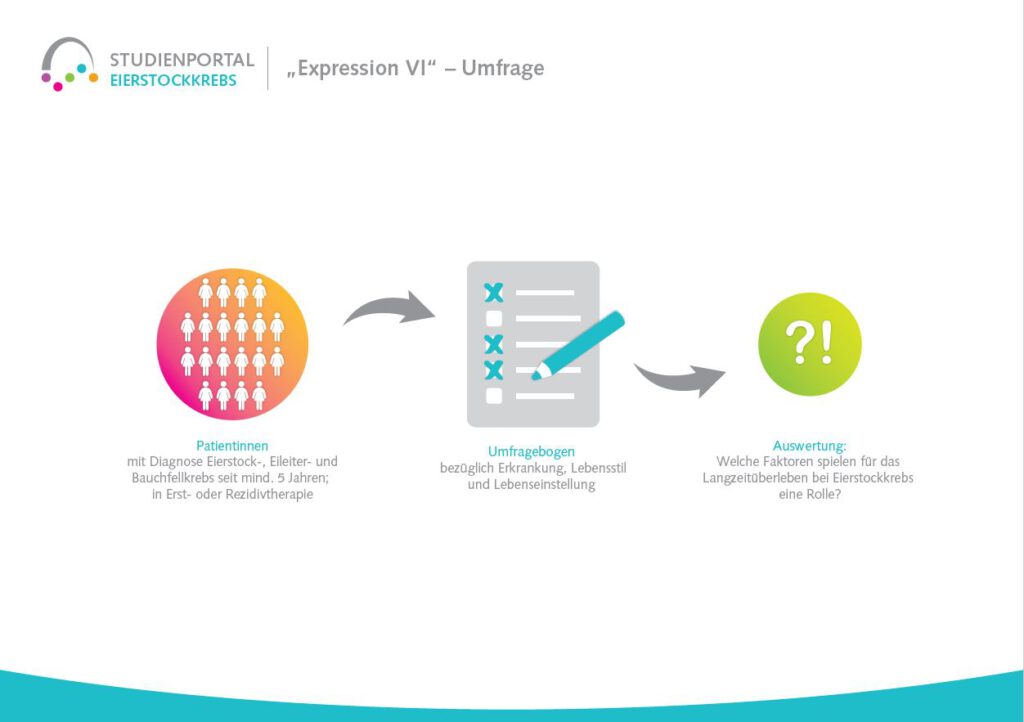
What is the survey process?
A total of 1000 patients will be surveyed internationally for the study. The survey is anonymous and the data will be collected using a questionnaire, which will be available both online and in paper form at the participating study centers.
The questionnaire comprises around 80 questions on fears of illness relapse, fears of crises, but also on media use, personal resilience and other relevant questions.
Are there any risks?
Expression 18 is a survey study and therefore has no participation risks.

Participation requirements
The survey is open to all patients over the age of 18 who have been diagnosed with gynecological cancer, including breast cancer, regardless of the stage of the disease and the treatments they have received.
Where can I take part in the survey?
Participation is now open online possible!
This page was updated on 27.01.2026